Quality System
The quality objectives of Unimed are to promote a flexible organisation and reactive management in order to satisfy entirely the requirements and demands of our clients. Unimed is certified since 1997 according to the applying industrial and medical standards and has established a quality management system based on two fundamental approaches:
- a systemic organisation
- a processus-oriented organisation
Our management and activities are organized to frame the product manufacturing cycle, from the submission of an offer to the shipment of the goods, and are integrally oriented towards our customer's needs.
Our quality management system is built on the structure of the international standard ISO 13485. It includes the requirements of the broader management system (ISO 9001, environmental, risk, safety and security) in order to comply with the legal and regulatory requirements.
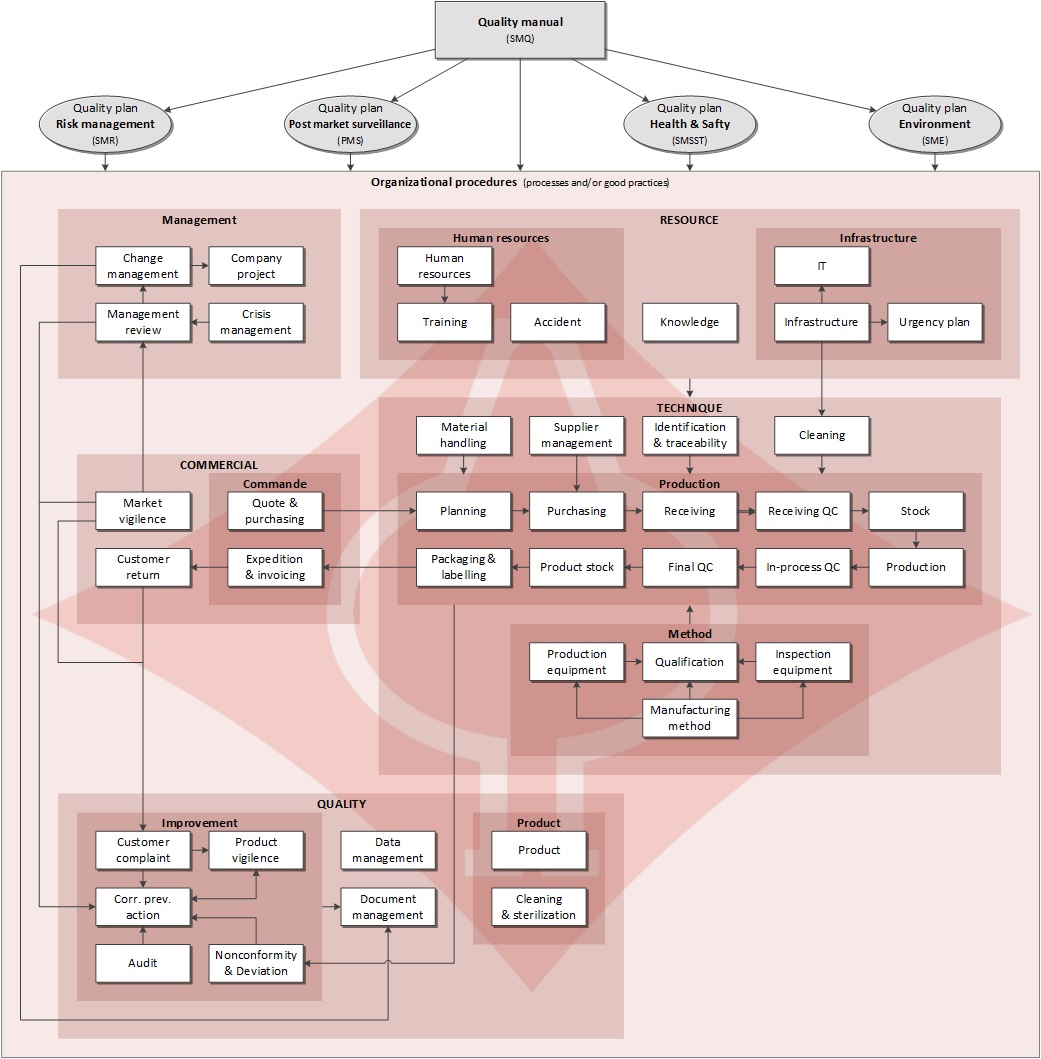
The quality management system is shown in our quality manual available from our Download Center page. Below is the cartography of the process and good organizational practices, defined to ensure Unimed's quality policy.
Compliance
Unimed's organisation is conform to the requirements of the ISO 13485 & 9001 quality standards and is FDA registered for the US market since 1979. Our certificate may be downloaded by clicking on the below corresponding logos or directly from our Downloads page.
Also, Unimed has integrated the specific requirements regarding health and environment threatening substances by fulfilling the requirements from the below directives and regulation :
- Regulation 1907/2006 - Restrictions concerning certain chemicals (REACH)
- Directive 2022/95/EC - Hazardous substances in electrical and electronic equipment (ROHS)
- Regulation n° 999/2001 - Transmissible Spongiform Encephalopathies (BSE-TSE)
- Certificate of compliance, Conflict Minerals